ZOLGENSMA is an essential,
one-time treatment option
Treated at ~4 months old
The only gene therapy and only one-dose treatment for SMA
ZOLGENSMA® (onasemnogene abeparvovec-xioi) is the only treatment that addresses the genetic cause of spinal muscular atrophy (SMA) with just one dose, and is a type of medicine called gene therapy. ZOLGENSMA works by increasing the amount of SMN protein in the body to keep the muscles working as they should. Patients will receive a corticosteroid before and after infusion with ZOLGENSMA and will undergo blood tests and monitoring.
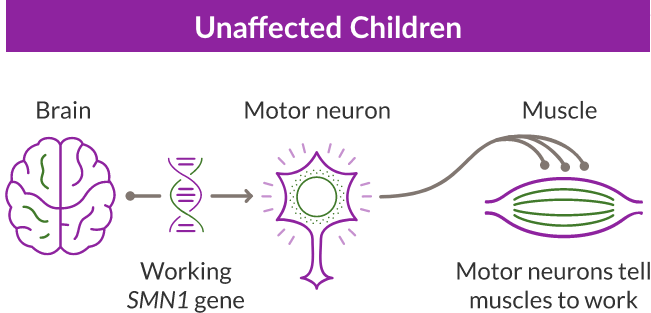
SMA is a progressive and rare genetic disease that affects the motor neuron cells in the spinal cord and impacts the muscles used for breathing, eating, crawling, and walking.
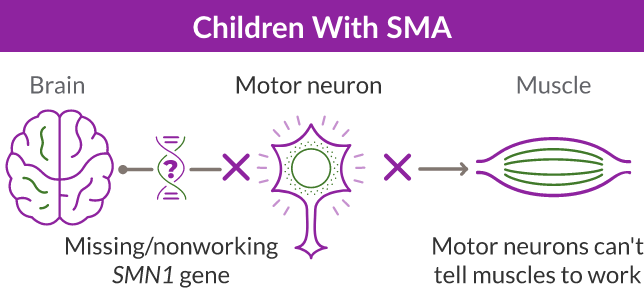
ZOLGENSMA is a gene therapy for SMA that replaces the function of the missing or nonworking SMN1 gene with a new, working SMN gene. The new SMN gene helps the body’s cells—particularly the motor neuron cells—to keep producing SMN protein.
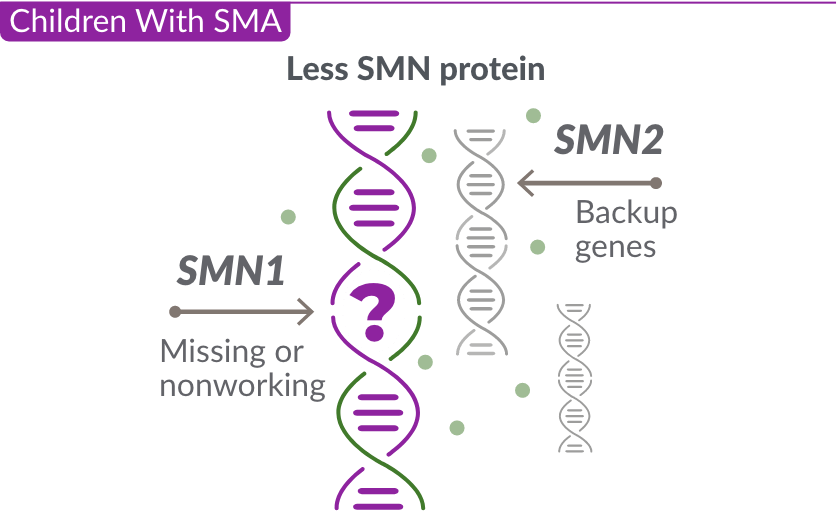
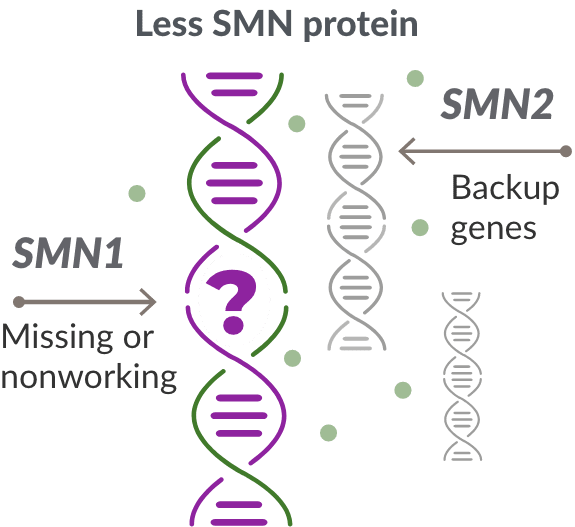
What causes SMA?
Missing or nonworking SMN1 gene
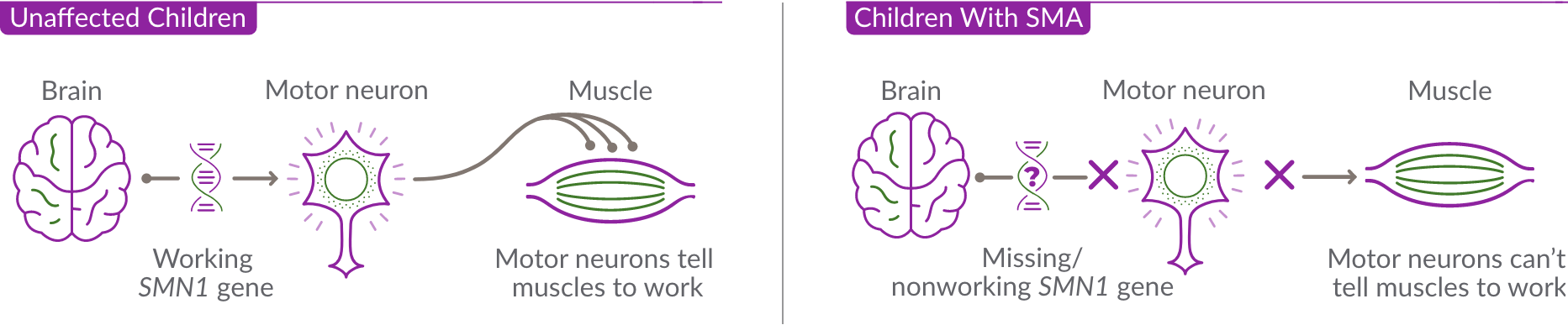
SMA is caused by a missing or nonworking survival motor neuron 1 (SMN1) gene. This is the primary gene responsible for producing the survival motor neuron (SMN) protein, which is critical to the health of motor neurons—the nerves that control our muscles. When the SMN1 gene is missing or is not working properly, the body cannot make enough SMN protein. Without enough SMN protein, some motor neuron cells throughout the body lose their ability to function and, as a result, die. When this happens, people with SMA may experience muscle weakness throughout the body, which can affect essential activities, such as walking, eating, and in some cases, breathing.
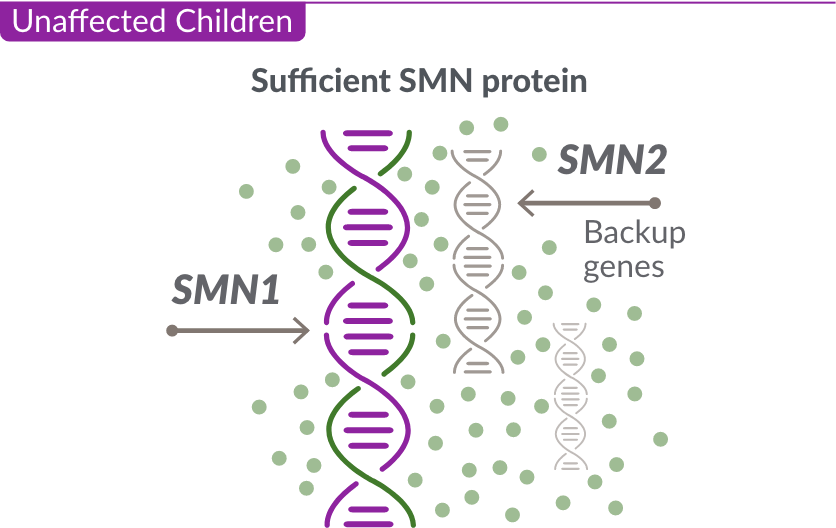
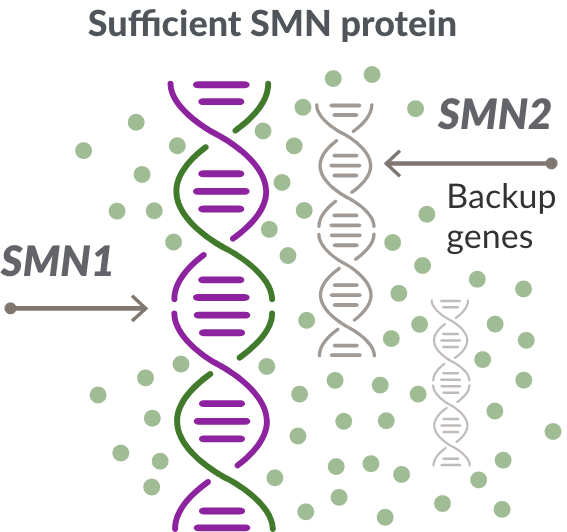
The role of the SMN2 backup gene
Like many genes, the SMN1 gene has a backup gene, called the survival motor neuron 2, or SMN2 gene. In people with SMA, when the SMN1 gene is missing or not working properly, the SMN2 gene can help produce SMN protein.
For people with SMA, the SMN2 gene is the main source of SMN protein production; however, it makes only about 10% of working SMN protein. ZOLGENSMA replaces the function of the missing or nonworking SMN1 gene.
Unaffected Children
Children With SMA
How ZOLGENSMA works
In SMA, the SMN1 gene is missing or not working properly. The SMN1 gene makes about 90% of the SMN protein your child needs.
ZOLGENSMA is the only gene therapy for SMA that replaces the function of the missing or nonworking SMN1 gene by delivering a new, working SMN gene to the body’s cells with a single infusion. ZOLGENSMA is engineered to keep continuously working in the body throughout your child’s lifetime.


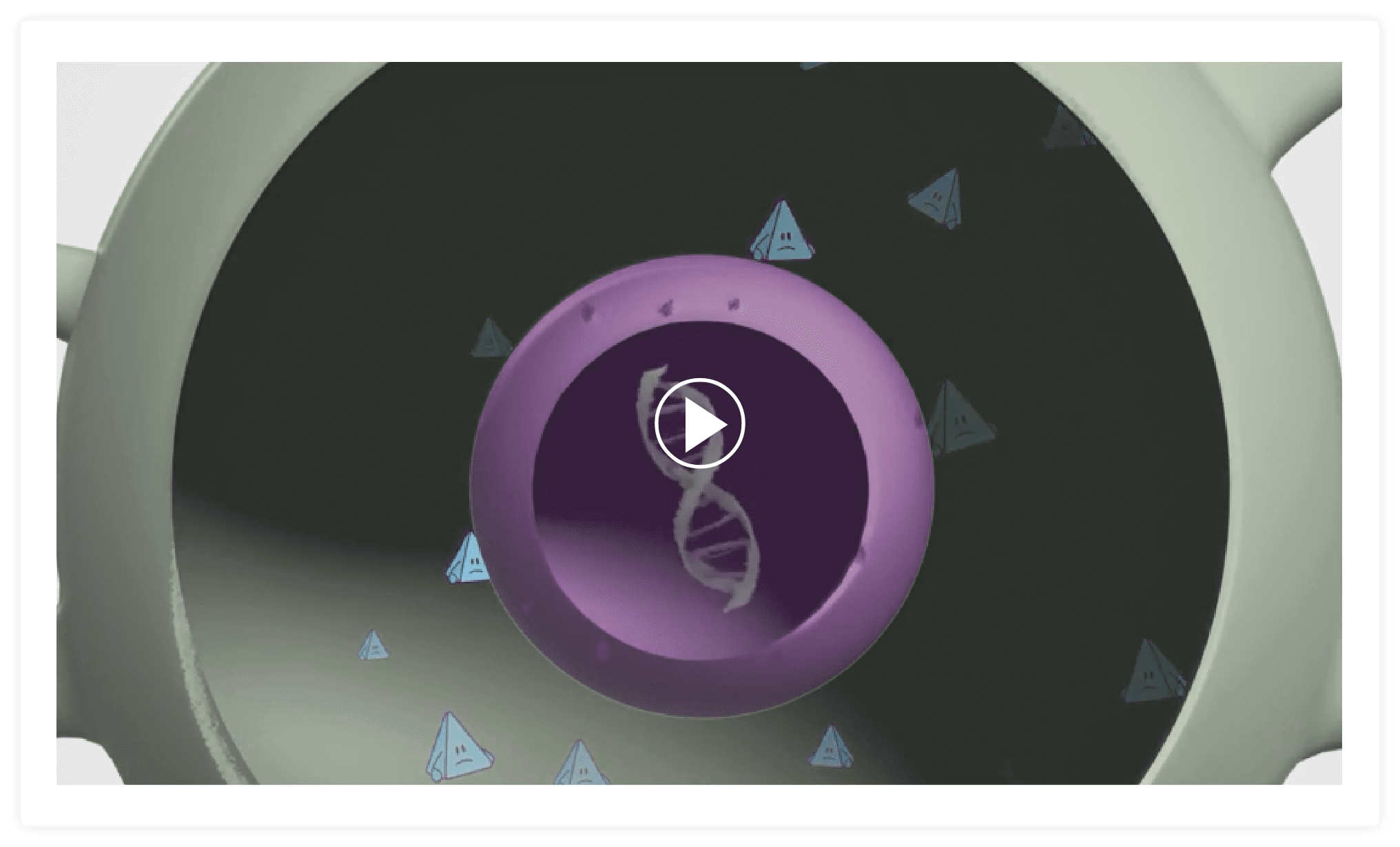
With ZOLGENSMA, a new, working SMN gene is placed inside a delivery vehicle called a vector.
The vector that delivers the SMN gene is made from a virus called AAV9. This type of virus is not known to make people sick. To make the vector, the DNA of the virus is removed so that the new SMN gene can be put inside.
ZOLGENSMA travels throughout the body and delivers the new, working gene to motor neuron cells.




By replacing the function of the SMN1 gene, ZOLGENSMA enables the production of SMN protein and helps preserve essential muscle function.
Motor neuron cells that would have died without treatment can survive and be maintained, stopping the progression of SMA.
Making a decision for a child with SMA?
Remember, only ZOLGENSMA is designed to work with a one-time dose.
See how ZOLGENSMA works
ZOLGENSMA is the only gene therapy that replaces the function of the SMN1 gene to stop the progression of SMA.